Find the latest news from Canteen featuring stories from the young people and families we support as well as updates on our research and services.

We're thrilled to announce that Canteen Australia is now on TikTok! This marks a significant milestone for us as we continue our mission to support young people when cancer turns their world upside down.

We surveyed young people who use Canteen Connect to understand what was working well and what we could improve.

Canteen Australia is pleased to announce the upcoming 6th Global Adolescent and Young Adult Cancer Congress, taking place on 3-6 December 2024 at the Melbourne Convention and Exhibition Centre.

Canteen has proudly launched its Culturally Responsive Framework. This framework is for all Canteen team members and outlines how Canteen will work with Aboriginal and/or Torres Strait Islander young people and their families impacted by cancer.

We’re delighted that the Australian Government has today announced $9.4m in funding to enable us to continue delivering this vital service until 2027.
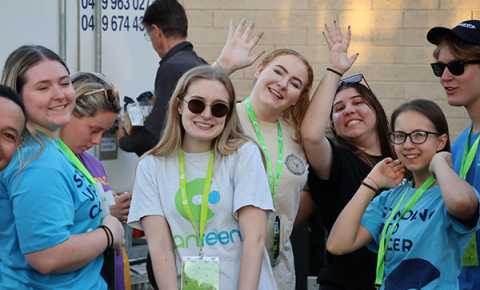
Relive the key moments from Life Cycle's 25 anniversary weekend in Western Australia. 3 days and 150 riders, all to support young people impacted by cancer.

Canteen teamed up with Delta Goodrem to deliver a special segment during Nine’s ‘Christmas with Delta’ that aired on 17, 24 & 25 December.

On Friday 2 February, Canteen young people and their families impacted by cancer got up close and personal with iconic Australian band The Living End for an exclusive, once-in-a-lifetime experience.

As we wrap up for the year, we're excited to share the remarkable achievements outlined in our Annual Report 2023.

Recently, Salt Meats & Cheese flung open their doors to our young people by hosting interactive cooking classes.

Canteen’s iconic National Bandanna Day has so far raised over $35 million to support young people impacted by cancer.
Support Canteen. Change lives.


You can help young people overcome the immense challenges of cancer in so many different ways from making a donation to donating your time.






